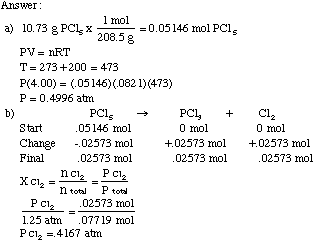
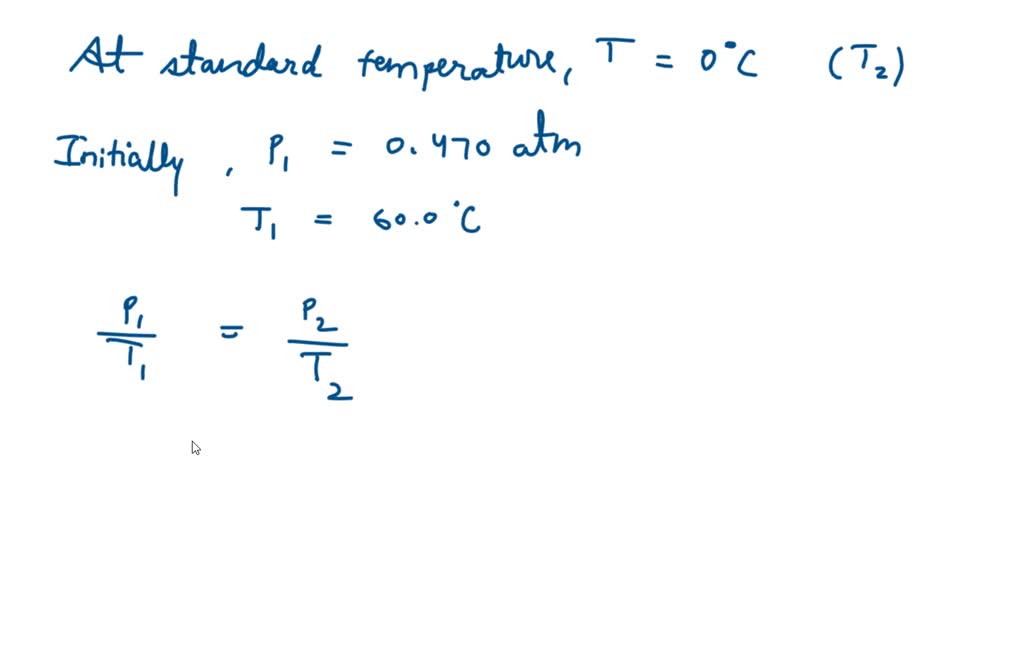Equivalents for the ways to measures of pressure Atmospheres (atm) Millimeters of mercury (mmHg) – Also known as TORR Kilopascals (kPa) 1 atm = 760 mmHg. - ppt download

At 546^oC and 1 atm pressure, 0.062 grams of gas occupies a volume of 33.6 ml . The molecular weight of the gas is:
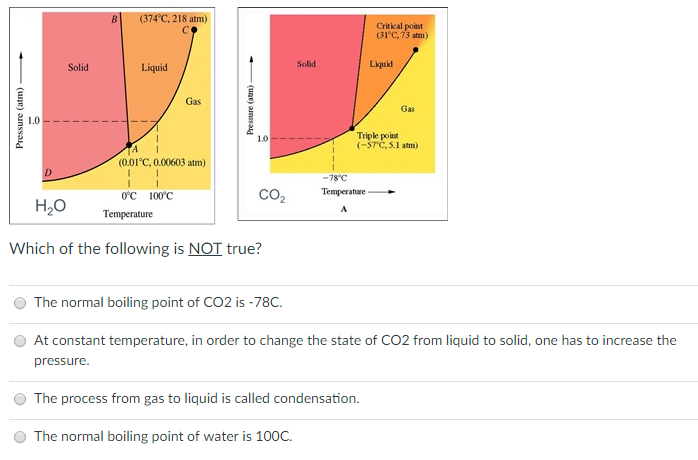
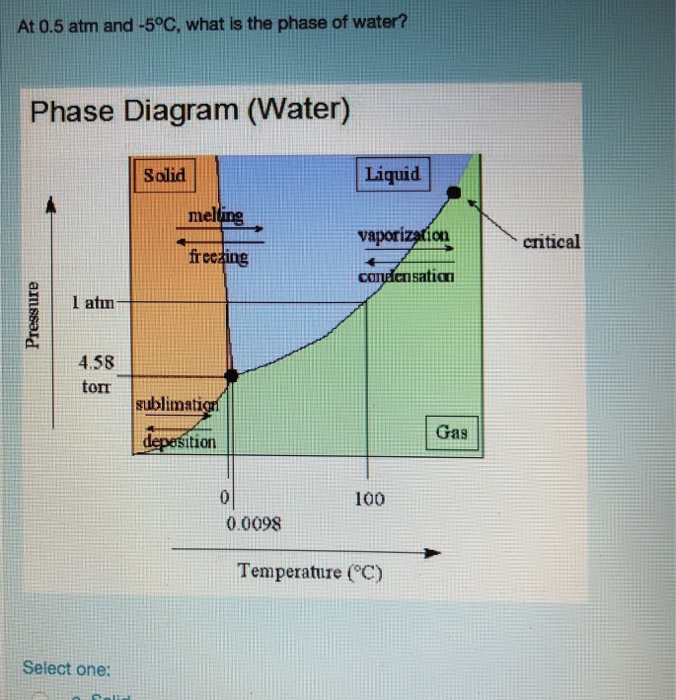
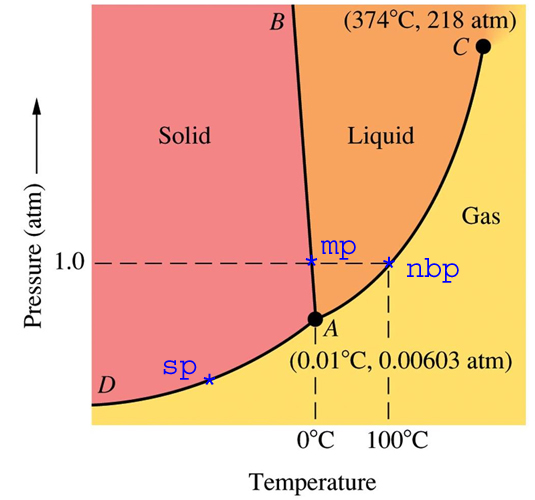
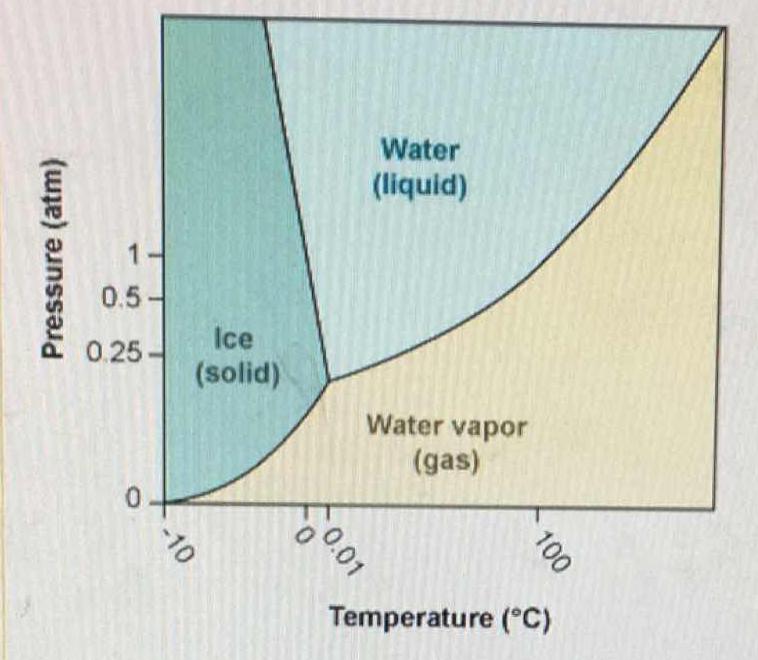
Why do 0°C and 1 atm can not be considered triple point for water? At 0°C and 1 atm, ice and water are in equilibrium and also they have water vapours due

In what phase is CO2 at 4 atm and -10 degrees Celsius? Is it a liquid, gas, or solid? | Homework.Study.com

A sample of a gas at 100^(@)C and 0.80 atm pressure has a density of 1.15 g L^(-1). What is the molecular weight of the gas?
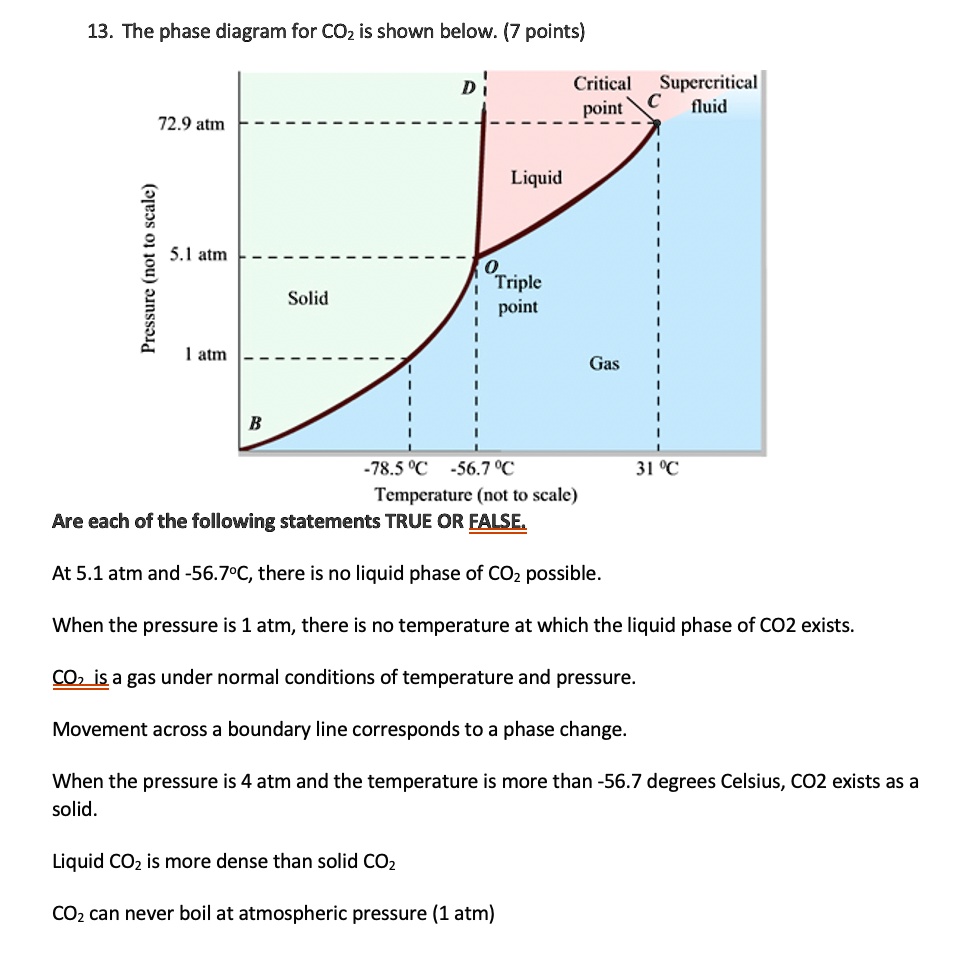
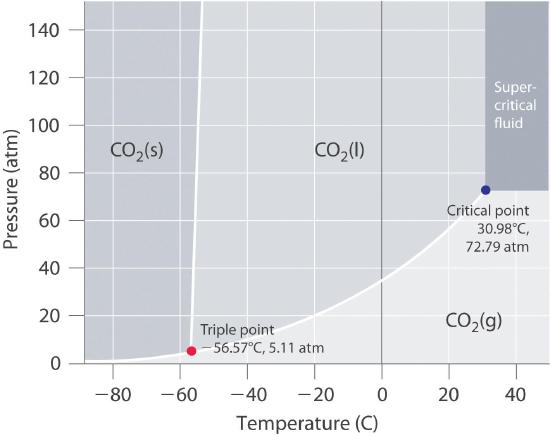
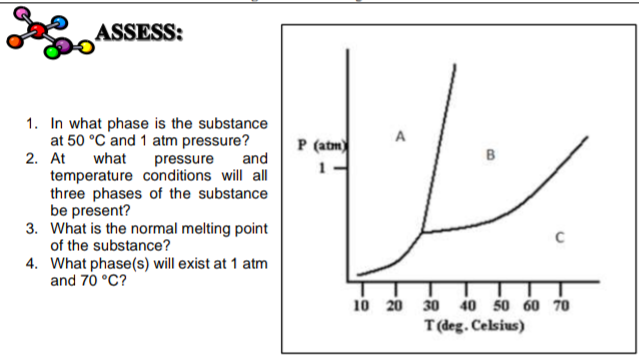
SOLVED: 13 The phase diagram for COz is shown below: points) Critical point Supereritical fluid 72.9 atm Liquid 1 1 5.1 atm L aln Triple point Solid Gas 78.5 %C 56.7 "€

Gas Pressure Unit Conversions - torr to atm, psi to atm, atm to mm Hg, kpa to mm Hg, psi to torr - YouTube
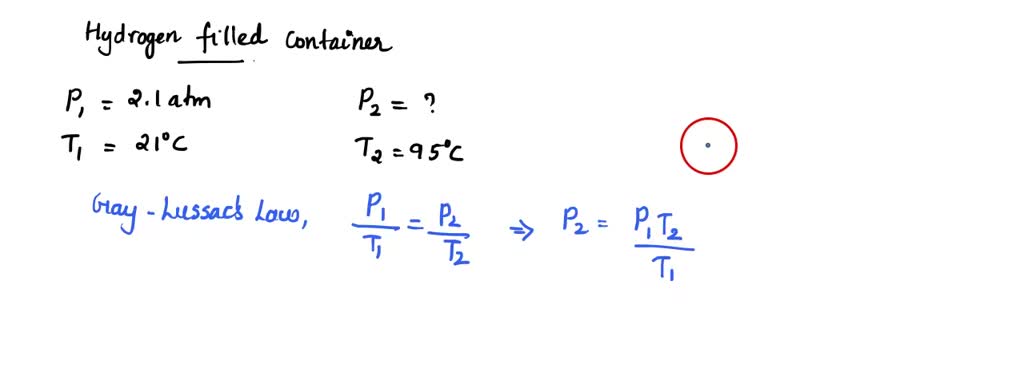
SOLVED: Part A The pressure inside hydrogen-filled container was 2.10 atm at 21 C. What would the pressure be if the container was heated to 95 "C ? Express your answer with

Which phase/s of water exist uder 0.35 atm and 71 degC? a. solid b. liquid c. gas d. supercritical fluid | Homework.Study.com

SOLVED: How is the pressure in container B related to the pressure in container A? 46. A container is filled with an ideal gas to a pressure of 40.0 atm at 0^∘C.